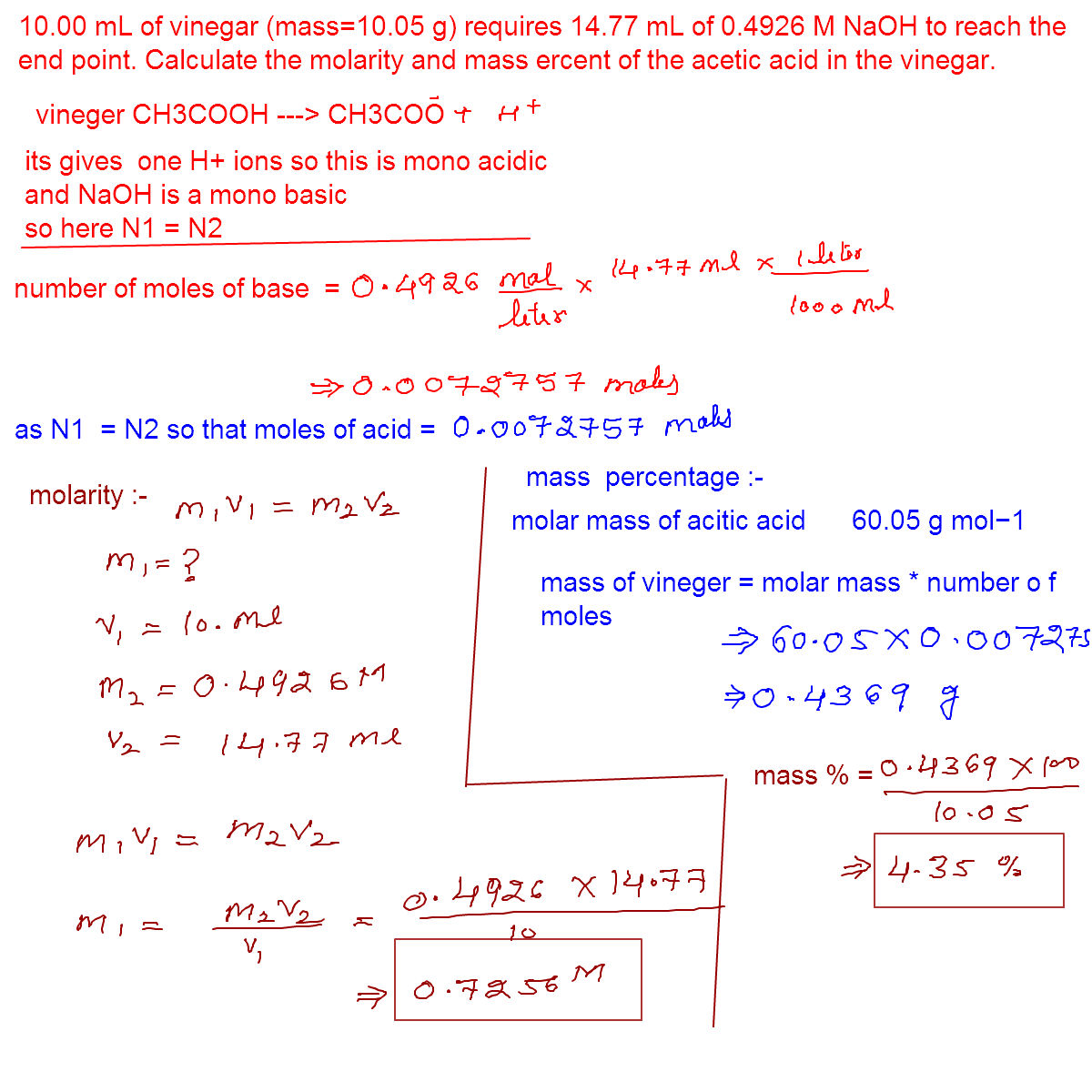
Online mathematics and chemistry help: 10.00 ml of vinegar (mass=10.05 Vinegar mass molarity requires ml naoh acid acetic percent calculate reach point end calculation help online Vinegar acetic calculate molarity numerade moles
Solved Assuming that the density of vinegar is 1.005 g/mL, | Chegg.com
Acid acetic vinegar mass calculate molar percent using naoh sample used yet molarity titration volume average buret reading trial total
Vinegar ml acid acetic calculate density average mass percentage molarity titration data naoh solved sample value answers using show volume
Solved analysis of vinegar trial 2 trial 1 volume of vinegarSolved:calculate the molarity (moles/l) of acetic acid in vinegar: use Solved a 2.70-l container of vinegar contains 3.3% (w/v) ofSolved assuming that the density of vinegar is 1.005 g/ml,.
.